Cloudbreak®
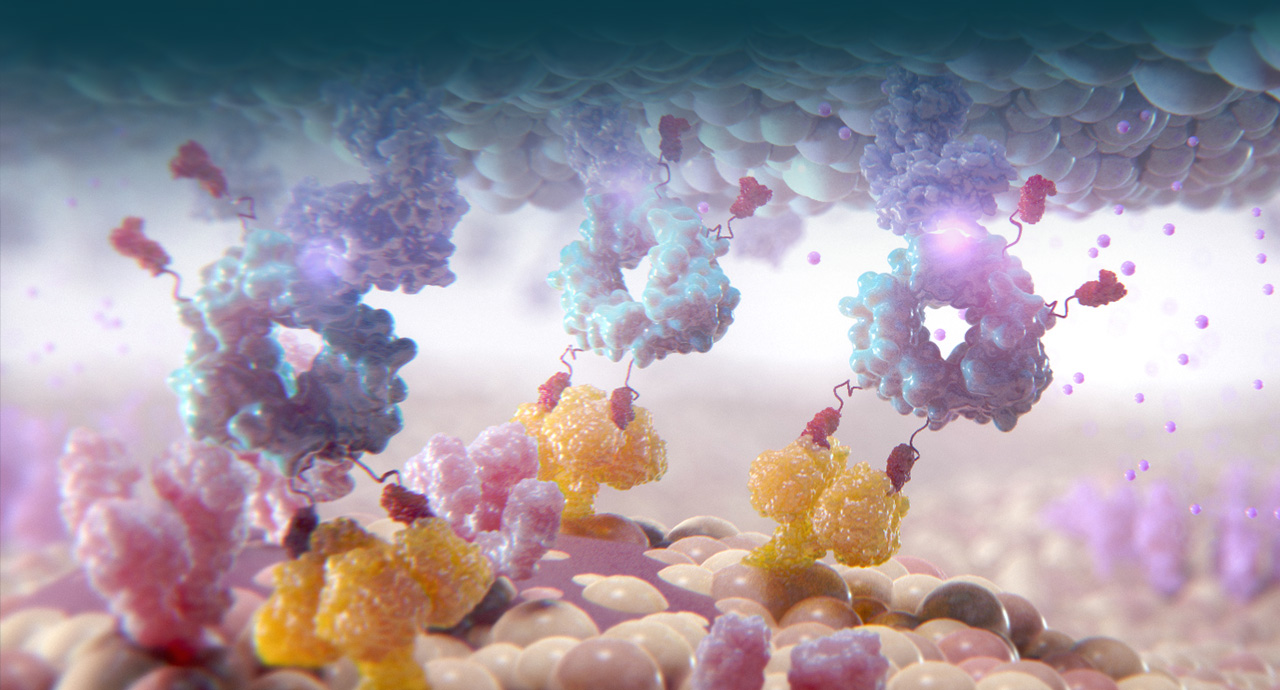
Cidara is using its proprietary Cloudbreak platform to develop drug-Fc conjugates (DFCs) that couple targeted small molecules and peptides to a human antibody fragment (Fc). These “single molecule cocktails” can be designed to inhibit specific disease targets and, when desired, to simultaneously engage the immune system.

Cidara’s Cloudbreak platform is a fundamentally new approach to preventing and treating serious diseases such as viral infections and tumors. By applying the principles of targeted immune modulation, Cidara is creating novel drugs that stably couple targeted small molecules or peptides, to a proprietary variant of a human antibody fragment (Fc). Structurally, these molecules are engineered to combine the best attributes of small molecules and antibodies and overcome the limitations of each. These agents can be designed to directly inhibit disease targets while simultaneously directing immune-mediated clearance of disease. The two distinct and complementary mechanisms are designed to provide potency and selectivity, while also providing an extended half-life and attracting an immune response to maximize disease eradicating activity.