Cloudbreak®
Cidara is using its proprietary Cloudbreak platform to develop drug-Fc conjugates (DFCs) that couple targeted small molecules and peptides to a human antibody fragment (Fc). These “single molecule cocktails” can be designed to inhibit specific disease targets and, when desired, to simultaneously engage the immune system.
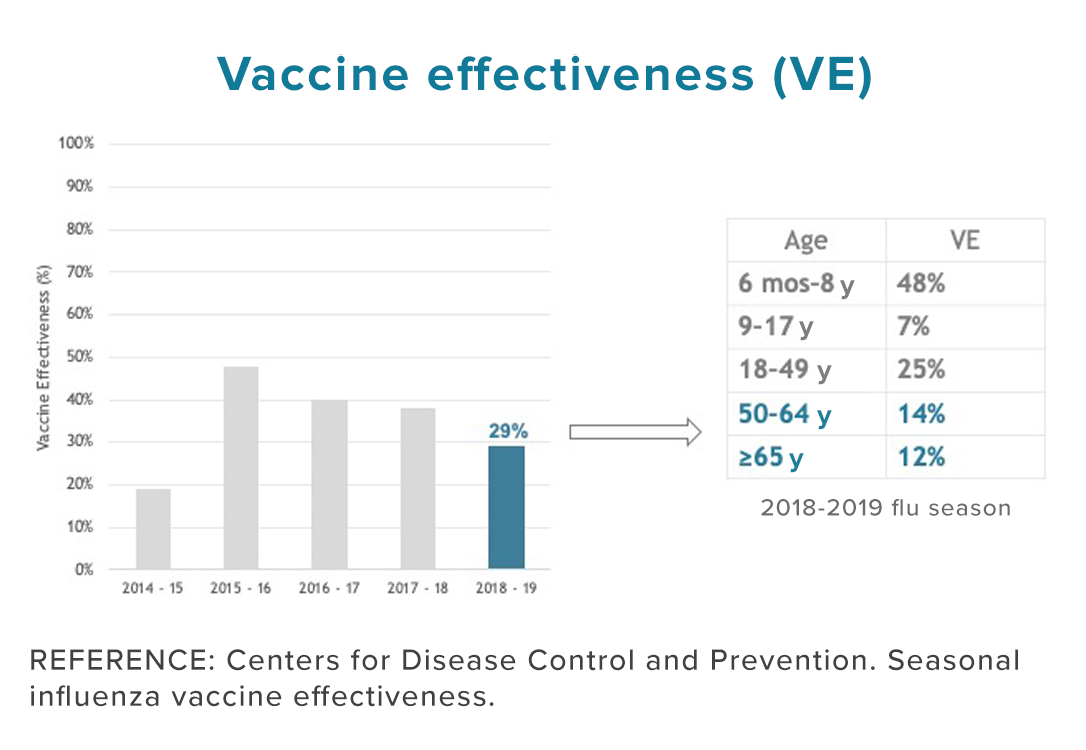
Influenza (“the flu”) is a contagious viral infection that can cause mild to severe illness, sometimes resulting in death. While today’s flu vaccines are credited with significant public health benefits and offer our current best defense, only 49% of Americans get an annual flu vaccine*, and a majority of those people do not respond. As a result, most Americans are still at risk of getting the flu yearly. In fact, during the 2021-22 flu season, a staggering 64% did not respond to vaccination and about 5,000 people died of flu-related illness in the US. The estimated average annual total economic burden of influenza to the healthcare system and society is more than $11.2 billion. For more information about influenza, visit the CDC website.
DFCs FOR INFLUENZA PREVENTION
Cidara is developing CD388, a flu DFC, to achieve universal prevention of seasonal and pandemic influenza with a single dose. DFCs are not vaccines or monoclonal antibodies. Their targeting domains are small molecule antivirals that bind to a highly conserved target on the influenza cell surface, which is essential for viral proliferation, enabling universal influenza coverage. These DFCs are designed to directly inhibit viral proliferation while simultaneously directing immune-mediated clearance of the virus. The two distinct and complementary mechanisms are designed to maximize antiviral activity of DFCs.
Flu DFCs have the potential to offer significant advantages over current flu vaccines:
- True universal protection, against all influenza strains for all people, including those with a compromised immune system
- Near-immediate protective effects
In a recent bioRxiv manuscript, CD388 demonstrated potent, universal activity across influenza A and B viruses, including high pathogenicity and neuraminidase resistant strains, a low potential for resistance development, and robust efficacy in lethal mouse infection models.
In March 2023, Cidara announced promising efficacy and safety data from a planned interim analysis of the Phase 2a trial evaluating the pre-exposure prophylactic activity of CD388 against the H3N2 influenza A virus strain (NCT05523089). The interim analysis was based on 56 subjects, with 28 receiving a single dose of CD388 (150 mg) and 28 receiving a placebo. Despite the small sample size, a decrease in viral replication in the upper respiratory tract and influenza infection was observed in participants receiving a single dose of CD388 when compared to placebo. Treatment with CD388 was generally well-tolerated, with no treatment emergent adverse events leading to study discontinuation or serious adverse events reported in the interim analysis. These promising data established preliminary clinical proof of concept for the ongoing CD388 development program.
* Calculated as a weighted average using census data as of July 2019 and flu vaccination coverage data from the US Centers for Disease Control