Cloudbreak®
Cidara is using its proprietary Cloudbreak platform to develop drug-Fc conjugates (DFCs) that couple targeted small molecules and peptides to a human antibody fragment (Fc). These “single molecule cocktails” can be designed to inhibit specific disease targets and, when desired, to simultaneously engage the immune system.
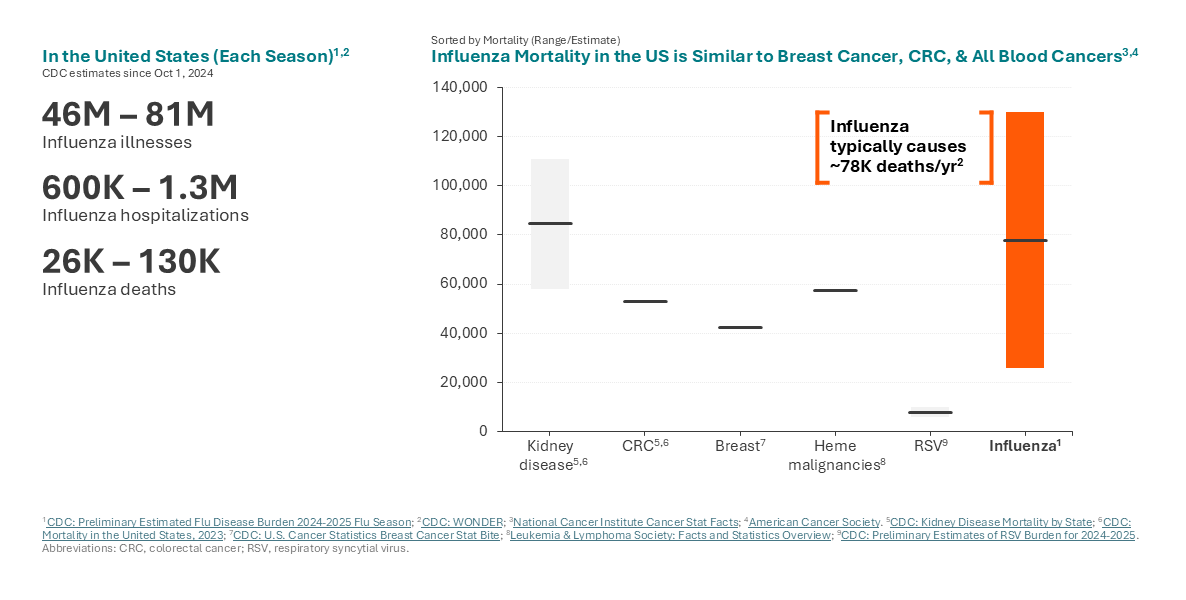
Influenza is a contagious viral infection that can cause mild to severe illness, sometimes resulting in death. While today’s flu vaccines are credited with significant public health benefits and offer our current best defense, influenza continues to drive significant morbidity and mortality. This is because not all people respond to the vaccine, especially those who are older or have compromised immune systems.
DFCs FOR INFLUENZA PREVENTION
Cidara is developing CD388, a flu DFC, that combines zanamivir, the active ingredient of FDA-approved influenza drug Relenza®, with a clinically-validated human antibody fragment. It is not a vaccine, but a long-acting antiviral drug. CD388 has the potential to be a single-dose, universal preventative against all flu strains.
CD388 aims to protect those at greatest risk of flu, such as those with compromised immune systems or high-risk comorbidities, as its efficacy does not depend on a functional immune system.
CD388 binds to a highly conserved target on the influenza cell surface, which is essential for viral proliferation, thereby inhibiting the flu virus from continuing to multiply and spread throughout the body.
Flu DFCs have the potential to offer significant advantages over current flu vaccines:
- True universal protection, against all influenza strains for all people, including those with a compromised immune system
- Near-immediate protective effects
In a recent publication in Nature Microbiology, CD388 demonstrated potent, universal activity across influenza A and B viruses, including high pathogenicity and neuraminidase resistant strains, a low potential for resistance development, and robust efficacy in lethal mouse infection models.
Cidara announced positive topline results from its 5,000+ person Phase 2b NAVIGATE clinical trial in June 2025 evaluating CD388 for the prevention of seasonal influenza in healthy unvaccinated adults. A single dose of the antiviral gave up to 76% protection from symptomatic influenza over 24 weeks compared to placebo. It was also well-tolerated with no safety signals observed. CD388 demonstrated statistically significant and clinically meaningful results, showing its potential to offer robust, once-per-season protection against influenza A and B.